At present, the main gas elements in cast iron are oxygen, nitrogen and hydrogen. The existing form and content of these three gas elements in molten iron have an important impact on the quality and performance of cast iron [1]. The influence of oxygen on the structure and properties of cast iron is mainly reflected in the solidification process of cast iron. Oxygen combines with various elements in molten iron to form oxides, one part of which forms graphite shaped nucleus and particle, and the other part forms oxide inclusions. Excessive oxygen will promote the oxidation of molten iron, reduce the fluidity of molten iron, and produce casting defects. At the same time, oxygen will consume additional inoculant spheroidizing agent in the process of inoculation spheroidization, resulting in poor inoculation spheroidization [2]. The influence of nitrogen on the structure and properties of cast iron is mainly reflected in that nitrogen can inhibit the growth of graphite flakes, shorten the length of graphite flakes, passivate the ends, increase the bending degree, and reduce the length width ratio [3]. An appropriate amount of nitrogen also plays a role in microalloying in cast iron. The solid solution strengthens the matrix structure, and improves the material properties. Excessive nitrogen will produce nitrogen pores [4]. Hydrogen has no good effect on the structure and properties of cast iron. According to research, the solubility of hydrogen in cast iron with carbon equivalent of 4.2% is 7.5 at 1200 ℃ × 10-6。 Excessive hydrogen is precipitated during solidification, resulting in the formation of hydrogen pinholes in castings.
In conclusion, in order to produce iron castings with stable performance and high quality, it is of great practical significance to detect the content of oxygen, nitrogen and hydrogen in iron castings and discuss the influence of their content on the structure and properties of iron castings.
1 Preparation and method of test materials
In this experiment, different types of gray cast iron and nodular cast iron were prepared by melting molten iron in medium frequency induction furnace, which was used to determine and analyze the content of oxygen and nitrogen in gray cast iron and nodular cast iron. The addition amount of scrap steel in the furnace charge is 50%~65%, the return charge is 20%~35%, and the pig iron is 0~20%. When preparing gray cast iron and nodular cast iron, semi graphitized carburetor and graphitized carburetor are respectively used for carburizing treatment. The addition amount is 1.0%~2.0%, and 0.6%~1.0% silicon carbide is added at the same time. When the temperature of molten iron rises to about 1450 ℃, samples shall be taken for composition analysis, and the * * * final molten iron shall be discharged at 1480-1520 ℃.
Preparation and analysis of samples. On site sampling: Φ Take samples with a 5 mm vacuum glass tube, insert the vacuum glass tube into the molten iron, immediately pump the iron bar into water, and put it into a clean plastic bag after cooling. The test bar taken out is dense and free of air holes. Laboratory sample preparation: remove the oxides on the surface of the sample by grinding, and immerse the sample in ethanol for cooling during the grinding process to avoid overheating. After grinding, put the sample in ethanol for storage. Use a hair dryer to dry the ethanol on the surface, and then use a diamond file to fine process the surface to make the surface of the sample smooth and free of rough lines. After * * *, process it into a sample (about 0.5g) suitable for the analysis amount for analysis.
During analysis, use tweezers to put the sample into the ANK ON-3000 analyzer for analysis. The results are the average values of 5 samples.
2. Determination of oxygen and nitrogen content in gray cast iron and nodular cast iron
After the grey cast iron is inoculated out of the furnace, the temperature drops to about 1350 ℃ for glass tube sampling, and the oxygen and nitrogen content are measured. The analysis results are shown in Table 1. In the samples HT250 and HT300, the internal oxygen content is generally much lower than the nitrogen content. Compared with the sample HT250, the mean values of oxygen content and nitrogen content of HT300 are higher.
Table 1 Oxygen and Nitrogen Content in Grey Cast Iron

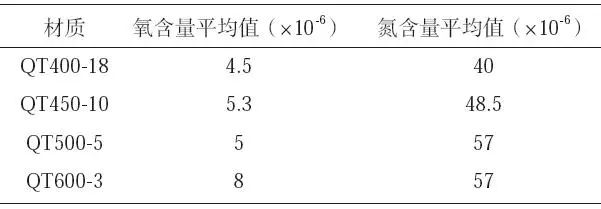
After the spheroidizing treatment of nodular cast iron, the temperature drops to about 1350 ℃ for glass tube sampling to measure the oxygen and nitrogen content. The analysis results are shown in Table 2. For different types of nodular cast iron, the internal oxygen content is far lower than the nitrogen content, and the mean difference of oxygen content is small, while the mean difference of nitrogen content is large. The mean value of nitrogen content in QT600-3 and QT500-5 is 57 × 10-6, the average nitrogen content of QT400-18 is * * * small, 40 × 10-6。
Effect of oxygen content on the quality of synthetic gray cast iron
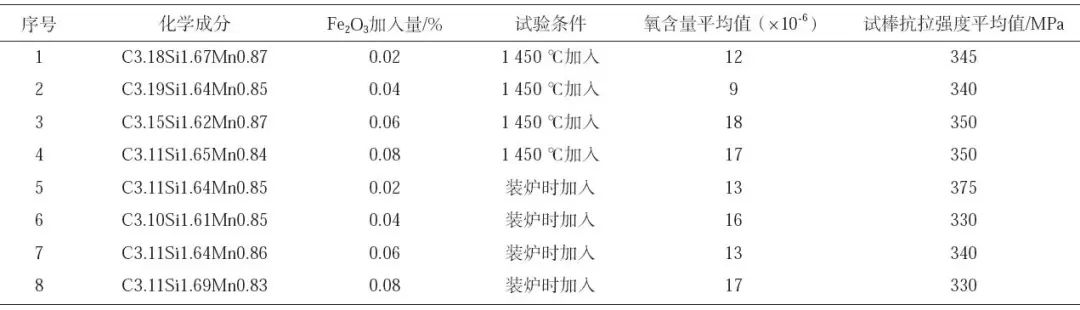
It can be seen from Table 3 that when Fe2O3 of different proportions is added at 1450 ℃ and during charging, its oxygen content is (12~18) × 10-6, average 13.75 × 10-6, the performance varies from 330 MPa to 375 MPa, without obvious regularity. It can be seen that the effect of artificially increasing the oxygen content with Fe2O3 is not obvious, and the oxygen content is not proportional to the tensile strength. However, the test component is HT300 material, and the strength has reached more than 330 MPa, with an average value of 347 MPa, indicating that the oxygen content in molten iron is (10-20) × 10-6. The reason is that when the oxygen content in molten iron is too low to 10 × When it is below 10-6, there are few oxides and sulfur oxygen compounds that can be used as the external core of graphite, and the molten iron is not responsive to inoculation treatment, so there will be more undercooled graphite (D, E type graphite) in the structure of gray cast iron. When the oxygen content is too high, additional alloy elements will be consumed, which will lead to the degradation of the properties of the sample.
4 Effect of Nitrogen Content on Microstructure and Properties of HT250
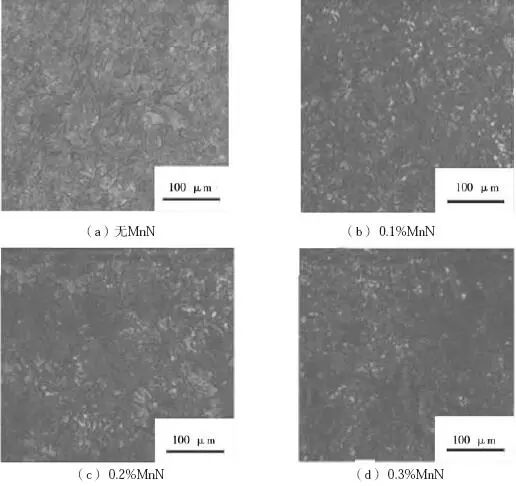
There are many discussions about the influence of nitrogen on the structure and properties of cast iron, and the understanding and utilization of nitrogen are more profound in all aspects. In this paper, the effect of nitrogen content on the microstructure and properties of gray cast iron was investigated by adding manganese nitride alloy to HT250 to increase the nitrogen content in molten iron. The test charge ratio is 60% for ordinary carbon scrap, 30% for homogeneous charge and 10% for pig iron. Add nitrided ferromanganese when molten iron is melted to 1450 ℃. The dosage is 0, 0.1%, 0.2% and 0.3% respectively. The test results are shown in Table 4. The results are the average values of 5 samples.
Table 4 Effect of MnN addition on microstructure and properties of cast iron
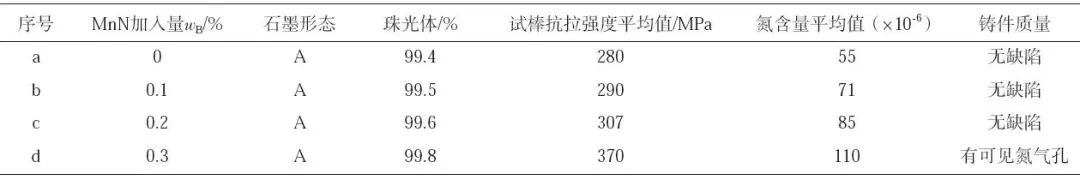
It can be seen from Table 4 that with the increase of MnN addition, the nitrogen content in gray cast iron is gradually increasing, the pearlite content is gradually increasing, the tensile strength of the sample is gradually increasing, and the graphite morphology in the cast iron is * * *. In addition, when the addition amount of MnN is 0.3%, the nitrogen content in gray cast iron is 110 × 10-6, the tensile strength of the sample can reach 370 MPa, but there are visible nitrogen pores in the casting (as shown at A, B, C in Figure 1).
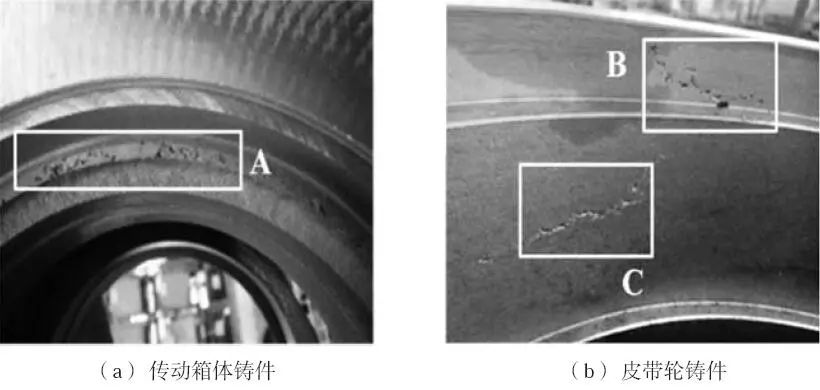
Fig. 1 Photo of nitrogen porosity defect

Fig. 2 Graphite morphology of nitrogen containing gray cast iron sample
Formation and Control of Hydrogen Porosity in QT450
The form of hydrogen in cast iron can be dissolved in a small amount in liquid or solid cast iron, or it can be separated as simple gas during solidification of cast iron, resulting in porosity defects in the casting [9]. During solidification of molten iron, it is difficult for hydrogen bubbles to float up and migrate inward. When they stay under the casting skin layer after * * *, they may cause porosity defects in the casting [10]. The hydrogen blowhole defect generally appears on the upper surface of the casting, with a round shape. There are many sources of hydrogen in the cast iron, mainly from the corrosion of furnace charge, furnace lining, cladding, mold coating and metal furnace charge surface. The oil stain is the main reason for the high hydrogen content in the molten iron. In addition, the drying and cleaning of the cladding is also the key factor for the hydrogen blowhole in the casting.
As shown in Figure 4, a lot of blowholes were found during the processing of the case cover with QT450 material, and the blowholes were hydrogen pinholes through testing. The main reason is that the lining of the first ladle of molten iron is not completely dried when pouring, and the alloy element aluminum in the molten iron reacts with water vapor to produce hydrogen, which forms hydrogen precipitation during the solidification of molten iron, thus producing hydrogen porosity defects in the castings. The reaction formula is:
2Al+3H2O→Al2+3H2↑
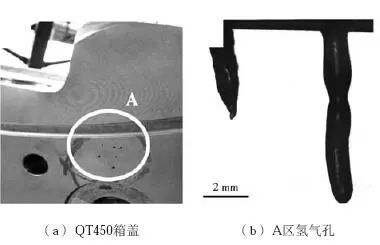
Photo 4 Defects of QT450 box cover and hydrogen gas hole